
Melting: Any cold substance absorbs energy. What Changes Occur Between Solids and Liquids?

The diagram shows the processes that describe how matter can change its state from solid to liquid, solid to gas, liquid to gas, liquid to solid, gas to solid, and gas to liquid.

The changing states of matter are always physical and commonly include: It depends on the energy supplied or absorbed by the substance in which form they are changing. What is the Reason Behind Changing States of Matter?Īlmost every substance undergoes a state change, whether it’s solid, liquid, or gas. When the temperature decreases, particles get a chance to relax into a more rigid structure. The effect of temperature on states of matter changing is directly proportional to the increase in interaction between the molecules present in the substance. Whenever there is a change in the pressure or temperature of a substance, changing states of matter occur. The diagram shows the effect of temperature on the states of matter and changes of state. However, in gases, particles are highly apart from each other and thus have an almost negligible force of attraction. In the liquid state, particles are quite separated from each other and hence have less force of attraction between them. In the solid-state, the molecules or particles are closely packed to each other, and hence they have a strong intermolecular force of attraction.
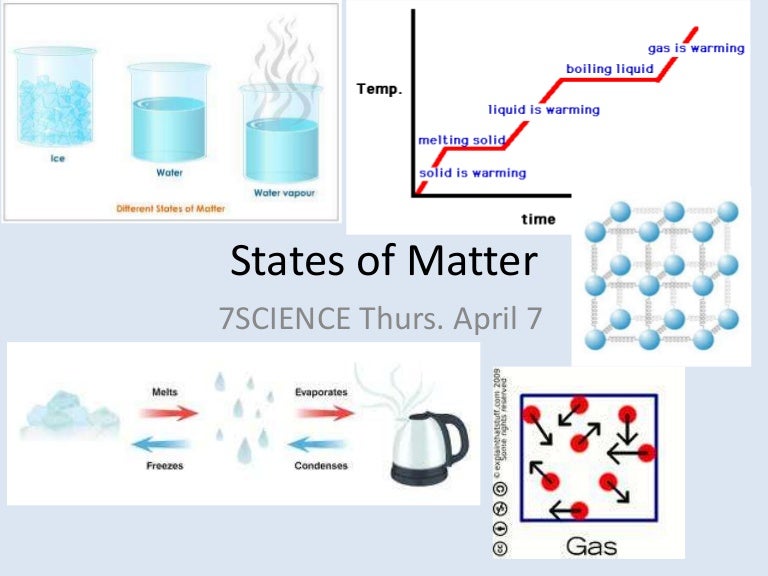
There are generally three states of matter: solid, liquid, and gas. After absorbing the energy, the atoms or molecules in the substance start moving rapidly, and the increased kinetic energy drives the particles far away. The reason behind such a change is the increase in kinetic energy. Whenever a substance absorbs energy or loses its energy, it changes its state. Have you ever wondered why ice turns into a liquid state after melting? Do you ever notice what happens when water boils? The answer to all such questions is straightforward: that is the changing states of matter.


 0 kommentar(er)
0 kommentar(er)
